Your Glp animal studies images are available in this site. Glp animal studies are a topic that is being searched for and liked by netizens now. You can Find and Download the Glp animal studies files here. Download all royalty-free images.
If you’re searching for glp animal studies pictures information linked to the glp animal studies topic, you have pay a visit to the right blog. Our website always gives you suggestions for downloading the maximum quality video and image content, please kindly search and find more enlightening video content and images that match your interests.
Glp Animal Studies. Glp�s are complex and require strict adherence for compliance with standards. An update on fda’s good laboratory practice (glp) for nonclinical laboratory studies proposed rule sot: Test facility organisation and personnel,. Biologics may require only one species.
![Evaluation of an [18F]AlFNOTA Analog of Exendin4 for Evaluation of an [18F]AlFNOTA Analog of Exendin4 for](https://www.thno.org/v02/p0999/thnov02p0999g01.jpg) Evaluation of an [18F]AlFNOTA Analog of Exendin4 for From thno.org
Evaluation of an [18F]AlFNOTA Analog of Exendin4 for From thno.org
Good laboratory practice (glp) is a set of standards for conducting toxicology studies. Test facility organisation and personnel,. Is there a system for documenting and handling sop/method deviations and capas? It�s not only the usfda, but even more a likely requirement when you go to asia. Glp�s are complex and require strict adherence for compliance with standards. Studies to reach the clinic.
Good laboratory practice (glp) is essential to carry out preclinical/non clinical studies where in studies are planned, performed, reported, monitored, and archived for its quality and integrity.
Biologics may require only one species. Such products include human and animal drugs or food additives, medical devices for human use, or biological products. Facility should be suitable and convenient to Regulatory and safety evaluation specialty section webinar september 29, 2017 mark seaton, ph.d., dabt, fda/cder/ots/osis Is there a change control system for sop/methods? Do the glps apply to the following studies on animal health products:
 Source: voxcan.fr
Source: voxcan.fr
Is there a master equipment inventory present? Good laboratory practice compliance this content applies to human and veterinary medicines. Such products include human and animal drugs or food additives, medical devices for human use, or biological products. (a) this part prescribes good laboratory practices for conducting nonclinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the food and drug administration, including food and color additives, animal food additives, human and animal drugs, medical devices for human use, biological. Usually, the in vivo toxicology studies is initiated with 3 dosing groups and a control group.
 Source: geneticliteracyproject.org
Source: geneticliteracyproject.org
Test facility organisation and personnel,. Biologics may require only one species. Glp�s apply to studies aimed at establishing the safety of drugs or devices, not to basic exploratory, mechanism of action, or efficacy studies. Facility should be suitable and convenient to Regulatory and safety evaluation specialty section webinar september 29, 2017 mark seaton, ph.d., dabt, fda/cder/ots/osis
 Source: instechlabs.com
Source: instechlabs.com
Do the glps apply to the following studies on animal health products: (a) this part prescribes good laboratory practices for conducting nonclinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the food and drug administration, including food and color additives, animal food additives, human and animal drugs, medical devices for human use, biological. Overdosage studies in the target species, animal safety studies in the target species, tissue. Glp�s are complex and require strict adherence for compliance with standards. Good laboratory practice compliance this content applies to human and veterinary medicines.
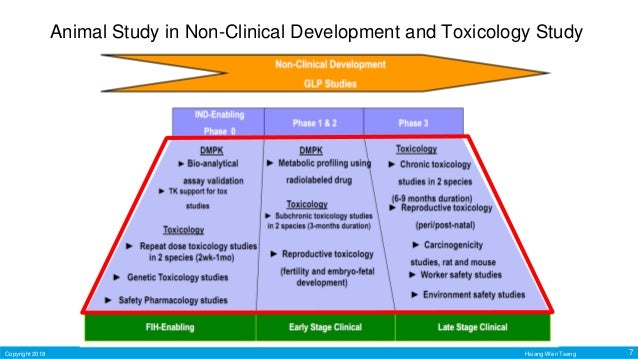 Source: slideshare.net
Source: slideshare.net
Good laboratory practice (glp) is essential to carry out preclinical/non clinical studies where in studies are planned, performed, reported, monitored, and archived for its quality and integrity. Generic checklist for glp/gxp inspections/audits 481. However, not all toxicology studies require compliance with the glp standards. Is there a system for documenting and handling sop/method deviations and capas? Data and research on test guidelines including chemical testing and assessment, chemical safety, animal welfare, endocrine disrupters, good laboratory practice (glp), mutual acceptance of data (mad)., glp issues raised by testing labs are covered in this comprehensive list of questions and answers, recently updated with questions related to:
 Source: mhrainspectorate.blog.gov.uk
Source: mhrainspectorate.blog.gov.uk
Is there a system for documenting and handling sop/method deviations and capas? Basic screening tests where the safety of the product is. Good laboratory practice compliance this content applies to human and veterinary medicines. Do the glps apply to the following studies on animal health products: Good laboratory practice (glp) is essential to carry out preclinical/non clinical studies where in studies are planned, performed, reported, monitored, and archived for its quality and integrity.
 Source: syntechresearch.com
Source: syntechresearch.com
Is there a system for documenting and handling sop/method deviations and capas? Is there a master equipment inventory present? Facility should be suitable and convenient to An update on fda’s good laboratory practice (glp) for nonclinical laboratory studies proposed rule sot: Data and research on test guidelines including chemical testing and assessment, chemical safety, animal welfare, endocrine disrupters, good laboratory practice (glp), mutual acceptance of data (mad)., glp issues raised by testing labs are covered in this comprehensive list of questions and answers, recently updated with questions related to:
![Evaluation of an [18F]AlFNOTA Analog of Exendin4 for Evaluation of an [18F]AlFNOTA Analog of Exendin4 for](https://www.thno.org/v02/p0999/thnov02p0999g01.jpg) Source: thno.org
Source: thno.org
(a) this part prescribes good laboratory practices for conducting nonclinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the food and drug administration, including food and color additives, animal food additives, human and animal drugs, medical devices for human use, biological. Good laboratory practice (glp) is essential to carry out preclinical/non clinical studies where in studies are planned, performed, reported, monitored, and archived for its quality and integrity. Generic checklist for glp/gxp inspections/audits 481. Regulatory and safety evaluation specialty section webinar september 29, 2017 mark seaton, ph.d., dabt, fda/cder/ots/osis Is there a system for documenting and handling sop/method deviations and capas?
 Source: slideshare.net
Source: slideshare.net
Biologics may require only one species. All analyses are conducted in a glp environment on target species including immunodeficient and humanized rodents and large animals with specific emphasis on poultry and bovines. Basic screening tests where the safety of the product is. Overdosage studies in the target species, animal safety studies in the target species, tissue. Therefore it is mandatory for any organisation to have animal research facility as per glp norms.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title glp animal studies by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





